JBRA Assist. Reprod. 2020;24(4):470-479
REVIEW
doi: 10.5935/1518-0557.20200014
Mining of variables from embryo morphokinetics, blastocyst’s morphology and patient parameters: an approach to predict the live birth in the assisted reproduction service
1Laboratório de Matemática Aplicada, Department of Biological Sciences, School of Languages and Sciences, Campus Assis, São Paulo State University (UNESP), Assis, SP, Brazil
2Laboratório de Micromanipulação Embrionária, Department of Biological Sciences, School of Sciences and Languages, Campus Assis, São Paulo State University (UNESP), Assis, SP, Brazil
CONFLICT OF INTERESTS
No conflict of interest has been declared.
ABSTRACT
Based on growing demand for assisted reproduction technology, improved predictive models are required to optimize in vitro fertilization/intracytoplasmatic sperm injection strategies, prioritizing single embryo transfer. There are still several obstacles to overcome for the purpose of improving assisted reproductive success, such as intra- and inter-observer subjectivity in embryonic selection, high occurrence of multiple pregnancies, maternal and neonatal complications. Here, we compare studies that used several variables that impact the success of assisted reproduction, such as blastocyst morphology and morphokinetic aspects of embryo development as well as characteristics of the patients submitted to assisted reproduction, in order to predict embryo quality, implantation or live birth. Thereby, we emphasize the proposal of an artificial intelligence-based platform for a more objective method to predict live birth.
Keywords: assisted reproductive technology, live birth prediction, artificial intelligence
INTRODUCTION
At the end of 1970s, in vitro fertilization (IVF) was an experimental process that resulted mostly in abortions or in unsuccessful pregnancies (Steptoe & Edwards, 1976) until, in July 1978, the first successful case obtained through IVF was the birth of Louise Brown (Steptoe & Edwards, 1978). After the introduction of intracytoplasmic sperm injection (ICSI) (Palermo et al., 1992), this technique was rapidly integrated into fertility clinics that offer assisted reproductive technology (ART) throughout the world. During the last years, ICSI has become the most frequently used method for fertilization, and in 2004, it was used in nearly 60% of all reported ART cycles in Australia and New Zealand, Europe and the USA (Wang et al., 2006; Wright et al., 2007; Andersen et al., 2008). In Latin America, in 2012, the largest number of assisted reproduction clinics and ART cycles performed were reported in Brazil, representing 45% of the total; followed by Argentina with 23% and Mexico with 12% (Zegers-Hochschild et al., 2014; 2015).
Although advances in IVF were responsible for refining the technology for the treatment of women with tubal disease, patients with natural or premature ovarian failure had no effective treatment until 1983 (Wang & Sauer, 2006). In the last decades, ART has continued to overcome a number of barriers that have allowed its constant improvement; however, singletons of ART pregnancies still exhibit increased maternal and neonatal complications (Zhu et al., 2016). In order to obtain a higher number of positive outcomes, the correct choice of the embryo to be transferred is fundamental to raise the number of live births (Ledford, 2018; Maheshwari et al., 2015). Initially, one of the options to obtain improvement of gestational rate was to transfer a high number of embryos, but it also led to increase in multiple pregnancy rate. To change this scenario, embryo selection by culture until blastocyst stage for the best one, which allows genomic activation and/or better endometrial synchronicity, associated with compaction and cavitation events of the embryonic cells, responsible for giving the blastocyst higher potential to establish a gestation has been performed (Rijnders & Jansen, 1998; Hardarson et al., 2012).
Since the selection of the best embryo to be transferred is such a defining milestone for gestational success, embryonic classification systems have been developed and improved over time. Among the analyzed morphological aspects are the number of cells, degree of compaction, fragmentation and size of the blastomeres (Lundin & Ahlström, 2015). The main morphological parameters analyzed are based on blastocyst stage and, currently, the most wide classification system used is based on morphological parameters graded by Gardner & Schoolcraft (1999), which involves expansion and hatching (EH) stage, from 1 (the least grade of expansion) to 6 (totally hatched); inner cell mass (ICM) grades, classified from A (many tightly packed cells) to C (very few cells) and trophectoderm (TE) grades, classified from A (many cells forming cohesive epithelium) to C (large and scarce cells). Despite the constant improvement of embryo selection systems, morphological evaluation observed through light microscopy remains the most used in assisted reproduction clinics worldwide (Alpha & ESHRE, 2011; Nasiri & Eftekhari-Yazdi, 2015; Puga-Torres et al., 2017).
As pointed by Alfarawati et al. (2011), most of the morphologic assessment criteria that are used to evaluate the embryo are only weakly correlated with IVF outcome. Embryo morphology is not always an absolute indicator for implantation potential once the best-looking blastocyst can fail to generate pregnancy or a morphologically suboptimal embryo can evolve into a healthy baby (Pribenszky et al., 2017). Furthermore, the nature of these evaluations and decisions made by embryologists is subjective, due to the existence of intra- and inter-observer variability (Sundvall et al., 2013), confounding (laboratory) factors such as differences in culture media and culture environment, beyond different handling of oocytes and embryos in the laboratory. Moreover, the quality of these assessments depends on the experience and attention to details, factor that is influenced by mood and fatigue at the moment of evaluation (Lundin & Ahlström, 2015; Rocha et al., 2016). Thereby, novel embryo selection technologies have emerged either to replace morphology-based embryo selection or to enhance conventional morphology-based embryo selection (Sengul et al., 2015).
The time-lapse system (TLS) allows embryo development monitoring without its remotion from the incubator, through a coupled camera and an appropriate software that produces a video recording of its evolution (Kovacs, 2014; Perkel et al., 2015). In addition, frames obtained by means of TLS allow the acquisition of morphological and kinetic information besides asymmetry of the cleavages in a non-invasive way (Wong et al., 2010; Chen et al., 2013; Milewski & Ajduk, 2017). The meticulous analysis of TLS images may also be used to detect potential embryo splitting signals, as detected in a monochorionic triamniotic pregnancy case, whereby there was elective single embryo transfer at the hatching blastocyst stage; thus, TLS can be a valuable tool to avoid the occurrence of multiple pregnancy (Sutherland et al., 2019).
Currently, there are many types of TLS available in the market. Some of them offer all items integrated in only one device, such as EmbryoScope® (Vitrolife), Geri (Genea Biomedx) and Miri® TL (Esco Medical). In contrast, other systems have the option of introducing a microscope into a regular incubator, e.g. Primo Vision® (Vitrolife) and the EevaTM Test (Merck-Serono) (Aparicio-Ruiz et al., 2018; Basile et al., 2019).
The ultimate purpose of TLS is selecting the embryo that has the best probability of resulting in live birth. Information obtained through TLS gives us knowledge about the morphological changes, kinetic and abnormalities on embryo that undergoes in vitro (Pribenszky et al., 2017). Therefore, the analysis of events that occur during embryonic development - the morphokinetic parameters - can be evaluated and, from this, algorithms may be produced to try to predict clinical outcomes of the embryo (Milewski & Ajduk, 2017).
Morphokinetic parameters can be evaluated for their capability to predict the quality of blastocyst (day 5). Table 1 depicts the main morphokinetic timings and their respective definitions. According to Storr et al. (2015), eight morphokinetic variables were considered predictive of top-quality blastocyst morphology (s3, t6, t7, t8, tM, tSB, tB and tEB). This observation contrasts with the results obtained by Cruz et al. (2012), whose morphokinetic parameters that indicated better morphological quality of the blastocyst were defined as t3, t5, s2 and cc2. At present, there are some published algorithms for the selection of embryos based on morphokinetic parameters, however frequently they can present incomplete datasets and/or heterogeneous methods that can result in conflicting results; hence, there is not one that is universally accepted (Barrie et al., 2017; Kaser & Racowsky, 2014; Aparicio-Ruiz et al., 2018).
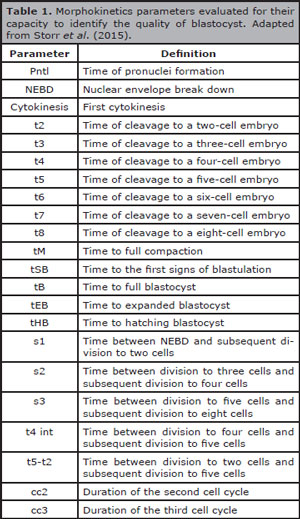
Table 1. Morphokinetics parameters evaluated for their capacity to identify the quality of blastocyst. Adapted from Storr et al. (2015).
Increased knowledge about the variables that influence the chance of success in ART may have a decisive impact on the guidance for using single embryo transfer (SET). Previous treatment of the patient, for instance, and the number of previous successful/failed IVF treatments were demonstrated to be of strong predictive value for live birth after IVF/ICSI (Nelson & Lawlor, 2011; van Loendersloot et al., 2014). Other than that, pre-pregnancy body mass index (BMI) significantly affects pregnancy outcomes, sometimes leading to gestational diabetes mellitus, gestational hypertension, preeclampsia, macrosomia, and caesarean delivery (Vesco et al., 2009; Baeten et al., 2001; Cedergren, 2004; Sebire et al., 2001; Bartolacci et al., 2019). Additionally, babies of obese mothers are more likely to experience prematurity, stillbirth, and congenital abnormalities (Provost et al., 2016). Currently, models for predicting live births after an IVF/ICSI cycle are under construction, some of them confirming embryo score, previous treatment, ovarian sensitivity, female age, endometrial thickness, infertility cause, and female height as independent predictors (Vaegter et al., 2017).
Although blastocyst formation and implantation rate are important markers of treatment efficacy, neither of them can be used to replace live birth rate or at least ongoing pregnancy (Kovacs, 2014). However, the own definition of live birth is controversial and Table 2 shows there is no consensus about the definition of this term.
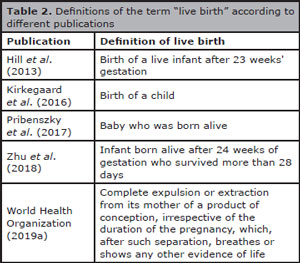
Table 2. Definitions of the term “live birth” according to different publications
The term known implantation data (KID) has been frequently employed in recent time-lapse researches as a developing and/or validating embryo selection algorithm in a dataset that comprehends a substantial number of double embryo transfer (DET) cycles (Liu et al., 2018; Basile et al., 2015; Liu et al., 2016; Meseguer et al., 2011). In these studies, embryos can be considered KID+ (positive) when refer to those generated from a SET cycle where a single fetal heartbeat is recognized under ultrasound or a DET cycle where two fetal heartbeats are recognized. On the other hand, KID- (negative) embryos are concerned to those from a cycle (both SET and DET) with a negative outcome unrelated to the number of embryos transferred. Besides that, KID data do not consider embryos from DET cycles with singleton pregnancy outcomes, due to the impossibility of knowing which embryo had implanted (Liu et al., 2018).
Despite the large range of information provided by the various international studies on assisted reproduction, a deeper analysis of the parameters that interfere in the gestational success and the birth of a healthy baby is still necessary. To that end, the constant evaluation and improvement of prediction models are fundamental, since the policies that encourage or determine the increased use of SET have been expanded in many countries, due to the risk of multiple gestations and maternal and neonatal complications (Karlström & Bergh, 2007). Therefore, our aim is to present several studies that have used morphological, morphokinetic, patient and partner variables submitted to ART and compare their results in order to predict live birth and/or fetal heartbeat. Based on these studies, we will propose an attempt to link the most relevant variables that contribute to the success of live birth in a way that can be reproducible, objective and non-invasive.
EMBRYO MORPHOLOGY AS PREDICTIVE PARAMETER
At the moment of the decision of which embryo to transfer during ART, morphology is still the most common parameter used for blastocyst quality evaluation (Reignier et al., 2018; Gardner et al., 2015). Therefore, in general, the best grades of the three morphological parameters (EH stage, TE and ICM qualities), will result in higher likelihood of live birth. However, sometimes there are no embryos in the best grades during the analysis and, even so, it is necessary to choose at least one for the transfer.
Thus, supported by studies based on fresh single blastocyst transfer, Ahlström et al. (2011) analyzed the independent ability of each morphological parameter to predict live birth, and all three parameters had significant effects on live birth rates, but the TE grade showed better predictive power compared to the other two parameters. Corroborating such results, Hill et al. (2013) showed that TE grade had the strongest correlation with live birth. Unlike both studies, Subira et al. (2016) found a strong correlation between ICM and live birth rate. In contrast, Du et al. (2016) evaluated the grade of expansion and re-expansion of the blastocoel as better ability to predict live birth (fresh cycles) and only the grade of blastocoel re-expansion was correlated with live birth in vitrified/warmed cycles.
EMBRYO MORPHOKINETIC AS PREDICTIVE PARAMETER
From the beginning of the use of TLS, which allows morphokinetic times evaluation, it is expected to be an improvement in embryologist's ability to select the embryo, which is most likely to be implanted, resulting in an improved clinical result (Kovacs, 2014).
Based on this, Goodman et al. (2016) evaluated the inclusion of specific kinetic parameters through TLS, in which results showed that the addition of time-lapse morphokinetic data did not significantly improve clinical reproductive outcomes. Besides that, the study indicated that only tSB was predictive of embryo implantation. On the other hand, Pribenszky et al. (2017) supported in their meta-analysis that time-lapse application was associated with a higher ongoing pregnancy rate and a significant increase on live birth rate. Reignier et al. (2019) analyzed the performance of KIDScore™ Day 5 morphokinetic prediction models and demonstrated a significant association with chances of pregnancy and live birth after blastocyst transfer, even though there may still be improvements.
Fishel et al. (2017) combined morphokinetic data in association with patient age and obtained an increase of 19% in the incidence of live births through embryo selection using morphokinetic algorithms for a cohort of patients younger than 38 years (using their own oocytes) and an increase of 37% for donated oocytes over 37 years.
PATIENT AND PARTNER VARIABLES AS PREDICTIVE PARAMETERS
It is widely accepted that fertility begins to decline years before the onset of menopause, even though continued regular ovulatory cycles happens, and that after 35 years, infertility becomes more frequent, a fact that deeply affects the chance of a full term birth of a baby (Van Noord-Zaadstra et al., 1991; Practice Committee of the American Society for Reproductive Medicine, 2006).
Several physiological characteristics of patients submitted to IVF are related with gestational success. Based on the analysis of more than 70,000 blastocysts, Acharya et al. (2017) found that advanced age, higher incidence of unexplained infertility, and high oocyte production were related to low blastulation rate. In contrast, high rate of blastulation correlated with lower number of oocytes recovered and with higher incidence of tubal factor infertility. These results challenge current knowledge that high oocyte yield leads to a higher number of blastocysts. Also, according to Almagor et al. (2015), the early embryos with irregular cleavage are significantly more prevalent in younger women.
Tan et al. (2014) reported a decrease of 13.2% in clinical pregnancy for women aged 40-44 years, compared to women less than 30 years. As concluded by Broekmans and Klinkert (2004), Lintsen et al. (2007) and Templeton et al. (1996), women age is considered the most important predictor of IVF success.
Thus, in a retrospective study conducted with 146 patients aged between 41 years to younger than 44 years who started the first IVF cycle attempt with their own oocytes, cumulative live birth rate was related to a decrease in the probability of live births with increasing age at the beginning of IVF treatment. Overall odds of a live birth rose up to 45% for women who started IVF at age 41, in contrast to 23% when the treatment started at age 43. Moreover, after 6 cycles of IVF, 42 patients (28.8%) gave birth a live infant (85.7% of the total live birth). The average rate of live births per cycle decreased with age in the initial cycle (8.0% at 41 years, 5.8% at 42 years and 4.1% at 43 years). In this study, patient age, smoking status and mean number of fertilized oocytes were considered as the major factors significantly correlated with the probability of a live birth (Lebovitz et al., 2018).
Decline in fertility with increasing age is due to the natural biological depletion of the ovarian reserve. The maximum pool of oocytes, approximately 6 to 7 million, exists during fetal life and undergoes decreasing during the course of pregnancy and drops to about 1-2 million by birth (Speroff, 1994). At puberty, 300,000 to 500,000 oocytes remain, and around the age of 37, when the rate of depletion doubles, it results in an increased rate of follicular loss, remaining only around 25,000 (Simpson, 2000). The number decreases to around 1,000 follicles when women achieves menopause (Navot et al., 1991; Loh et al., 2005). Also, Tan et al. (2014) found a doubling in miscarriage rate, increasing from 15.1% among women less than 30 years to 30.0% among those with 38 years, leading to the conclusion that another factor linked to the lower live birth rate with increasing maternal age are obstetric complications, mainly due to aneuploidy.
Another retrospective study analyzed the influence of BMI on live birth through IVF, considering the BMI criteria of the World Health Organization (2019b), according to Table 3 Table 3. Low BMI was correlated with decrease in live birth rates and increase in miscarriage rates compared to normal weight, controlling for covariates that influence the treatment outcome; nonetheless, the age of the patients was the most potent confounder variable. Moreover, low BMI had a more profound effect on live birth rates in patients older than 35 years, whereas the effect in younger patients was insignificant (Cai et al., 2017). The inverse was also analyzed and demonstrated the declined probability of live birth following IVF in obese patients in comparison to normal weight patients (Sermondade et al., 2019).
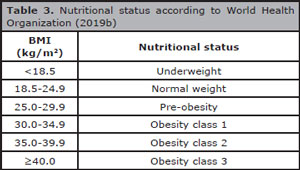
Table 3. Nutritional status according to World Health Organization (2019b)
Although van Swieten et al. (2005) reported that obesity is negatively associated with IVF/ICSI outcome, Fedorcsák et al. (2004) reported a significant linear association between higher BMI (above 30 kg/m²) and increased dose and longer stimulation with follicle-stimulating hormone (FSH), increased frequency of cycle cancellation, lower number of oocytes retrieved and lower number of embryos transferred. It was also associated with increased incidence of pregnancy loss before the sixth week of gestation, increased miscarriage during 6 to 12 weeks of pregnancy, lower live birth and cumulative live birth rates. Esinler et al. (2008) pointed that obese women had a higher risk of cycle cancellation due to poor ovarian response and lower fertilization rates.
Independent of chronological age, ovarian ageing affects both oocyte fecundity and quality and can negatively impact on the outcome of ART (Akande et al., 2002; Alviggi et al., 2009). With ovarian ageing, the diminishing proportion of normal oocytes needs to be compensated for quantitatively by increasing the number of available oocytes through controlled ovarian hyperstimulation. The result is the ovarian response as one of the parameters most commonly studied and reported in clinical research on IVF treatment, e.g. aiming to seek measures to optimize live births rate and minimize the risk of an increase in ovarian hyperstimulation syndrome (Li et al., 2014; Fiedler & Ezcurra, 2012). Baseline tests, such as serum FSH, inhibin B, estradiol and anti-Müllerian hormone (AMH), clomiphene citrate test and antral follicle count (AFC) correlate with the degree of ovarian response, but with limited accuracy in relation to the prediction of pregnancy (Broekmans et al., 2006; Pettersson et al., 2010).
AMH is a glycoprotein produced by granulosa cells of small and large preantral and small antral follicles (La Marca et al., 2010; Weenen et al., 2004). This hormone is secreted during the early follicular stage by follicles up to 6 mm in diameter and is also relatively independent of gonadotropin and remains relatively constant within and between menstrual cycles (Fanchin et al., 2005; Hehenkamp et al., 2006; La Marca et al., 2006; Tsepelidis et al., 2007; Van Disseldorp et al., 2010; Rasool & Shah, 2017). AMH levels peak at 25 years and gradually decline thereafter (Garcia-Velasco et al., 2005; Grossman et al., 2008; Durlinger et al., 2001). AMH declines years before the visible increase in FSH levels, thus being a more sensitive biomarker of the ovarian reserve (Freeman et al., 2012a; 2012b; Rasool & Shah, 2017). However, a generalized limit of AMH to predict pregnancy outcomes does not exist, since oocyte quality is not accounted for solely by quantitative ovarian reserve markers (Wang et al., 2010; Broekmans et al., 2006).
Templeton et al. (1996), and Nelson & Lawlor (2011), through the analysis of IVF and ICSI cycles, have identified predictors of live birth following IVF, pointing female age, duration of infertility and previous pregnancy as key prognostic factors. Templeton et al. (1996) showed a significant reduction in the success rate with the increasing duration of infertility, and a higher live birth rate per embryo transfer on women with unexplained infertility than woman with other causes. For Nelson & Lawlor (2011), the odds of successful live birth also decreased with increasing maternal age, increasing duration of infertility, greater number of previously unsuccessful IVF treatments, when the woman's own oocytes was used, and when this was the second or third treatment cycle, being lower when the cause of infertility was tubal, anovulatory, or cervical disease or when it was due to a male cause.
Among the results obtained by Templeton et al. (1996), stands out that the best possibility of success is in the first cycle of IVF treatment and that there is a significant negative effect with increasing number of attempts thereafter. Besides that, results show that the chance of a live birth begins to fall rapidly after 4 previous unsuccessful cycles, suggesting an inverse relationship between the success of IVF and the number of prior unsuccessful attempts (Nelson & Lawlor, 2011; Roberts et al., 2010).
Male infertility is also a factor which can influence the live birth rates. According to a study that considered 781 men with average total testosterone (TT) of 411 (318-520) ng/dL, those with TT<264 ng/dL were less likely to have normal morphology sperm and the chances of live birth decreased by 40% in couples whose male partner had low TT. As follows, the study pointed out that low TT in the male partner was associated with abnormal sperm morphology and lower live birth rates (Trussell et al., 2019).
FUTURE PERSPECTIVES
A promising tool for predicting embryo quality, gestational success and live birth based on one or more parameters, such as those described above (Table 4), has been highlighted by its powerful predictive potential: the artificial intelligence (AI) techniques; which have already been used, through digital image processing and artificial neural networks, to classify the quality of mammalian embryos based on the morphological aspects of the blastocyst stage (Rocha et al., 2016; Matos et al., 2014; Rocha et al., 2017).
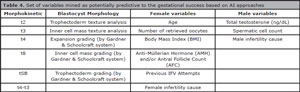
Table 4. Set of variables mined as potentially predictive to the gestational success based on AI approaches
Recent researches involving human embryos and AI can also be highlighted: Miyagi et al. (2019a), through machine learning approaches, used 160 blastocyst images that were implanted to develop a method for classifying embryos to predict the probability of reaching live birth, obtaining as result 65% of accuracy. In other study using specifically deep learning techniques, Miyagi et al. (2019b) based on blastocyst images to predict the probability of live birth in patients classified by age, used a total of 5,691 blastocyst images and the best accuracies obtained were 81% and 88%, respectively for the ages 40-41 and ≥ 42 years.
Blank et al. (2018) also used machine learning approaches to predict implantation potential after single blastocyst transfer, combining morphological characteristics associated with patient variables, e.g. parental age, AMH concentration and number of oocytes. Their application resulted in area under the curve (AUC) of 0.74.
Moreover, Khosravi et al. (2019) proposed a computational method based in deep learning techniques, to predict the quality of human embryos. Their approach used 10,148 digital images, obtained by TLS, and it could predict the morphological quality of blastocysts with accuracy of 98%. Also, through a deep learning model, Tran et al. (2019) predicted the probability of pregnancy with fetal heartbeat from TLS, based on 10,638 human embryos, and obtained an AUC of 0.93.
CONCLUSION
In view of all the variables shown in this review, which have the potential to predict live birth after ART, it is clear that many attempts have been produced to reduce the subjectivity of the conventional morphological embryonic evaluation. However, from the analysis of the previously described studies, it can be noted that in the majority - if not in full - of them, they do not use many of the variables that can predict live birth (Table 4). This observation could be a factor of why some of them have not achieved satisfactory results.
The approach of the AI techniques offers an outlet for this problem, once this tool allows the application of several variables as input to the software and obtain as output the probability of live birth. Nonetheless, to our knowledge, there is still no available software that includes the main variables that influence the success of full-term birth: morphological and morphokinetic embryo parameters, patient and partner clinical characteristics. As pointed by Simopoulou et al. (2018), the development of a program that includes such variables depends on the availability of a large database with KID results. Thus, we emphasize the possibility of developing a platform based on AI that includes all these variables as input and can predict the probability of live birth as output, in a way that is objective, accurate and with high reproducibility. Finally, this platform may be integrated in an app that facilitates its clinical use for embryologists and medical professionals in general.
ACKNOWLEDGEMENTS
We would like to acknowledge the following grants #2012/50533-2, #2017/19323-5 and #2018/19053-0 São Paulo Research Foundation (FAPESP) and #47956 (PIBIC Reitoria from São Paulo State University - UNESP).
REFERENCES
Acharya KS, Jones C, Keyhan S, Acharya CR, Muasher SJ. How do patient and in vitro fertilization (IVF) cycle characteristics impact blastulation rates? An analysis of 70,968 blastocyst cycles from the SART registry. Fertil Steril. 2017;108:e92. DOI: 10.1016/j.fertnstert.2017.07.285
Crossref
Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289-96. PMID: 21972253. DOI: 10.1093/humrep/der325
Medline Crossref
Akande VA, Fleming CF, Hunt LP, Keay SD, Jenkins JM. Biological versus chronological ageing of oocytes, distinguishable by raised FSH levels in relation to the success of IVF treatment. Hum Reprod. 2002;17:2003-8.
Medline Crossref
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520‐4.
Medline Crossref
Almagor M, Or Y, Fieldust S, Shoham Z. Irregular cleavage of early preimplantation human embryos: characteristics of patients and pregnancy outcomes. J Assist Reprod Genet. 2015;32:1811-5.
Medline Crossref
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270-83.
Medline Crossref
Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101.
Medline Crossref
Andersen AN, Carlsen E, Loft A. Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update. 2008;14:593-604.
Medline Crossref
Aparicio-Ruiz B, Romany L, Meseguer M. Selection of preimplantation embryos using time-lapse microscopy in in vitro fertilization: State of the technology and future directions. Birth Defects Res. 2018;110:648-53.
Medline Crossref
Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91:436-40.
Medline Crossref
Barrie A, Homburg R, McDowell G, Brown J, Kingsland C, Troup S. Examining the efficacy of six published time-lapse imaging embryo selection algorithms to predict implantation to demonstrate the need for the development of specific, in-house morphokinetic selection algorithms. Fertil Steril. 2017;107:613-21.
Medline Crossref
Bartolacci A, Buratini J, Moutier C, Guglielmo MC, Novara PV, Brambillasca F, Renzini MM, Dal Canto M. Maternal body mass index affects embryo morphokinetics: a time-lapse study. J Assist Reprod Genet. 2019;36:1109-16.
Medline Crossref
Basile N, Elkhatib I, Meseguer M. A Strength, Weaknesses, Opportunities and Threats analysis on time lapse. Curr Opin Obstet Gynecol. 2019;31:148-55.
Medline Crossref
Blank C, Wildeboer RR, DeCroo I, Tilleman K, Weyers B, de Sutter P, Mischi M, Schoot BC. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2018;111:318-26.
Medline Crossref
Broekmans FJ, Klinkert ER. Female age in ART: when to stop? Gynecol Obstet Invest. 2004;58:225-34.
Medline Crossref
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
Medline Crossref
Cai J, Liu L, Zhang J, Qiu H, Jiang X, Li P, Sha A, Ren J. Low body mass index compromises live birth rate in fresh transfer in vitro fertilization cycles: a retrospective study in a Chinese population. Fertil Steril. 2017;107:422-9.e2.
Medline Crossref
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-24.
Medline Crossref
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035-43.
Medline Crossref
Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25:371-81.
Medline Crossref
Du QY, Wang EY, Huang Y, Guo XY, Xiong YJ, Yu YP, Yao GD, Shi SL, Sun YP. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil Steril. 2016;105:910-9.e1.
Medline Crossref
Durlinger ALL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Tremmen AP. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891-9.
Medline Crossref
Esinler I, Bozdag G, Yarali H. Impact of isolated obesity on ICSI outcome. Reprod Biomed Online. 2008;17:583-7.
Medline Crossref
Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Müllerian measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923-7.
Medline Crossref
Fedorcsák P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, Omland AK, Abyholm T, Tanbo T. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19:2523-8.
Medline Crossref
Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012;10:32.
Medline Crossref
Fishel S, Campbell A, Montgomery S, Smith R, Nice L, Duffy S, Jenner L, Berrisford K, Kellam L, Smith R, D'Cruz I, Beccles A. Live births after embryo selection using morphokinetics versus conventional morphology: a retrospective analysis. Reprod Biomed Online. 2017;35:407-16.
Medline Crossref
Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimüllerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril. 2012a;98:1254-9.e1-2.
Medline Crossref
Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012b;97:1673-80.
Medline Crossref
Garcia-Velasco JA, Moreno L, Pacheco A, Guillén A, Duque L, Requena A, Pellicer A. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82-7.
Medline Crossref
Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21:727-47.
Medline Crossref
Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-85.e10.
Medline Crossref
Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364-70.
Medline Crossref
Hardarson T, Van Landuyt L, Jones G. The blastocyst. Hum Reprod. 2012;27:i72-91.
Medline Crossref
Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian Hormone levels in spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-63.
Medline Crossref
Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, Browne PE, Levens ED. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99:1283-9.e1.
Medline Crossref
Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20:617-31.
Medline Crossref
Karlström PO, Bergh C. Reducing the number of embryos transferred in Sweden-impact on delivery and multiple birth rates. Hum Reprod. 2007;22:2202-7.
Medline Crossref
Khosravi P, Kazemi E, Zhan Q, Malmsten JE, Toschi M, Zisimopoulos P, Sigaras A, Lavery S, Cooper LAD, Hickman C, Meseguer M, Rosenwaks Z, Elemento O, Zaninovic N, Hajirasouliha I. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit Med. 2019;2:21.
Medline Crossref
Kirkegaard K, Sundvall L, Erlandsen M, Hindkjær JJ, Knudsen UB, Ingerslev HJ. Timing of human preimplantation embryonic development is confounded by embryo origin. Hum Reprod. 2016;31:324-31.
Medline Crossref
Kovacs P. Embryo selection: the role of time-lapse monitoring. Reprod Biol Endocrinol. 2014;12:124.
Medline Crossref
La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103-7.
Medline Crossref
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16:113-30.
Medline Crossref
Lebovitz O, Haas J, James KE, Seidman DS, Orvieto R, Hourvitz A. The expected cumulative incidence of live birth for patients starting IVF treatment at age 41 years or older. Reprod Biomed Online. 2018;37:533-41.
Medline Crossref
Ledford H. IVF at 40: revisiting the revolution in assisted reproduction. Nature; 2018. Available at: https://www.nature.com/articles/d41586-018-05792-9.
Crossref
Li HW, Lee VC, Ho PC, Ng EH. Ovarian sensitivity index is a better measure of ovarian responsiveness to gonadotrophin stimulation than the number of oocytes during in-vitro fertilization treatment. J Assist Reprod Genet. 2014;31:199-203.
Medline Crossref
Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertil Steril. 2016;105:656-62.e1.
Medline Crossref
Liu Y, Feenan K, Chapple V, Matson P. Assessing efficacy of day 3 embryo time-lapse algorithms retrospectively: impacts of dataset type and confounding factors. Hum Fertil (Camb). 2018;22:182-90.
Medline Crossref
Lintsen AM, Eijkemans MJ, Hunault CC, Bouwmans CA, Hakkaart L, Habbema JD, Braat DD. Predicting ongoing pregnancy chances after IVF and ICSI: a national prospective study. Hum Reprod. 2007;22:2455-62.
Medline Crossref
Loh FH, Khin LW, Saw SM, Lee JJ, Gu K. The age of menopause and the menopause transition in a multiracial population: a nation-wide Singapore study. Maturitas. 2005;52:169-80.
Medline Crossref
Lundin K, Ahlström A. Quality control and standardization of embryo morphology scoring and viability markers. Reprod Biomed Online. 2015;31:459-71.
Medline Crossref
Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30:2703-7.
Medline Crossref
Matos FD, Rocha JC, Nogueira MF. A method using artificial neural networks to morphologically assess mouse blastocyst quality. J Anim Sci Technol. 2014;56:15.
Medline Crossref
Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658-71. PMID: 21828117. DOI: 10.1093/humrep/der256
Medline Crossref
Milewski R, Ajduk A. Time-lapse imaging of cleavage divisions in embryo quality assessment. Reproduction. 2017;154:R37-53.
Medline Crossref
Miyagi Y, Habara T, Hirata R, Hayashi N. Feasibility of artificial intelligence for predicting live birth without aneuploidy from a blastocyst image. Reprod Med Biomol. 2019a;18:204-11.
Medline Crossref
Miyagi Y, Habara T, Hirata R, Hayashi N. Feasibility of deep learning for predicting live birth from a blastocyst image in patients classified by age. Reprod Med Biomol. 2019b;18:190-203.
Medline Crossref
Nasiri N, Eftekhari-Yazdi P. An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell J. 2015;16:392-405.
Medline Crossref
Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocytes quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375-7.
Medline Crossref
Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilization: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8:e1000386.
Medline Crossref
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17-8.
Medline Crossref
Perkel KJ, Tscherner A, Merrill C, Lamarre J, Madan P. The ART of selecting the best embryo: A review of early embryonic mortality and bovine embryo viability assessment methods. Mol Reprod Dev. 2015;82:822-38.
Medline Crossref
Pettersson G, Andersen AN, Broberg P, Arce JC. Pre-stimulation parameters predicting live birth after IVF in the long GnRH agonist protocol. Reprod Biomed Online. 2010;20:572-81.
Medline Crossref
Practice Committee of the American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86:S248-52.
Medline Crossref
Pribenszky C, Nilselid AM, Montag M. Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta-analysis. Reprod Biomed Online. 2017;35:511-20.
Medline Crossref
Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril. 2016;105:663-9.
Medline Crossref
Puga-Torres T, Blum-Rojas X, Blum-Narváez M. Blastocyst classification systems used in Latin America: is a consensus possible? JBRA Assist Reprod. 2017;21:222-9.
Medline Crossref
Rasool S, Shah D. Fertility with early reduction of ovarian reserve: the last straw that breaks the Camel's back. Fertil Res Pract. 2017;3:15.
Medline Crossref
Reignier A, Girard JM, Lammers J, Chtourou S, Lefebvre T, Barriere P, Freour T. Performance of Day 5 KIDScoreTM morphokinetic prediction models of implantation and live birth after single blastocyst transfer. J Assist Reprod Genet. 2019;36:2279-85.
Medline Crossref
Reignier A, Lammers J, Barriere P, Freour T. Can time-lapse parameters predict embryo ploidy? A systematic review. Reprod Biomed Online. 2018;36:380-7.
Medline Crossref
Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869-73.
Medline Crossref
Roberts S, McGowan L, Hirst WM, Brison DR, Vail A, Lieberman BA. Towards single embryo transfer? Modelling clinical outcomes of potential treatment choices using multiple data sources: predictive models and patient perspectives. Health Technol Assess. 2010;14:1-237.
Medline Crossref
Rocha JC, Passalia F, Matos FD, Maserati MP Jr, Alves MF, Almeida TG, Cardoso BL, Basso AC, Nogueira MFG. Methods for assessing the quality of mammalian embryos: How far we are from the gold standard? JBRA Assist Reprod. 2016;20:150-8.
Medline Crossref
Rocha JC, Passalia FJ, Matos FD, Takahashi MB, Ciniciato DS, Maserati MP, Alves MF, de Almeida TG, Cardoso BL, Basso AC, Nogueira MFG. A Method Based on Artificial Intelligence To Fully Automatize The Evaluation of Bovine Blastocyst Images. Sci Rep. 2017;7:7659.
Medline Crossref
Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175-82.
Medline Crossref
Sengul YA, Bener AB, Uyar A. Emerging technologies for improving embryo selection: a systematic review. Adv Health Care Technol. 2015;1:55-64.
Crossref
Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, Fréour T. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:439-51.
Medline Crossref
Simopoulou M, Sfakianoudis K, Maziotis E, Antoniou N, Rapani A, Anifandis G, Bakas P, Bolaris S, Pantou A, Pantos K, Koutsilieris M. Are computational applications the “crystal ball” in the IVF laboratory? The evolution from mathematics to artificial intelligence. J Assist Reprod Genet. 2018;35:1545-57.
Medline Crossref
Smith ADAC, Tilling K, Nelson SM, Lawlor DA. Live-Birth Rate Associated With Repeat In Vitro Fertilization Treatment Cycles. JAMA. 2015;314:2654-62.
Medline Crossref
Speroff L. The effect of aging on fertility. Curr Opin Obstet Gynecol. 1994;6:115-20.
Medline
Steptoe PC, Edwards RG. Reimplantation of a human embryo with subsequent tubal pregnancy. Lancet. 1976;1:880-2.
Medline Crossref
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366.
Medline Crossref
Storr A, Venetis CA, Cooke S, Susetio D, Kilani S, Ledger W. Morphokinetic parameters using time-lapse technology and day 5 embryo quality: a prospective cohort study. J Assist Reprod Genet. 2015;32:1151-60.
Medline Crossref
Subira J, Craig J, Turner K, Bevan A, Ohuma E, McVeigh E, Child T, Fatum M. Grade of the inner cell mass, but not trophectoderm, predicts live birth in fresh blastocyst single transfers. Hum Fertil (Camb). 2016;19:254-61.
Medline Crossref
Sundvall L, Ingerslev HJ, Breth Knudsen U, Kirkegaard K. Inter- and intra-observer variability of time-lapse annotations. Hum Reprod. 2013;28:3215-21.
Medline Crossref
Sutherland K, Leitch J, Lyall H, Woodward BJ. Time-lapse imaging of inner cell mass splitting with monochorionic triamniotic triplets after elective single embryo transfer: a case report. Reprod Biomed Online. 2019;38:491-6.
Medline Crossref
Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J. 2014;55:305-9.
Medline Crossref
Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilization treatment. Lancet. 1996;348:1402-6.
Medline Crossref
Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal pregnancy following time-lapse incubation and blastocyst transfer. Hum Reprod. 2019;34:1011-8.
Medline Crossref
Trussell JC, Coward RM, Santoro N, Stetter C, Kunselman A, Diamond MP, Hansen KR, Krawetz SA, Legro RS, Heisenleder D, Smith J, Steiner A, Wild R, Casson P, Coutifaris C, Alvero RR, Robinson RB, Christman G, Patrizio P, Zhang H, Lindgren MC; Reproductive Medicine Network. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil Steril. 2019;111:1129-34.
Medline Crossref
Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-40.
Medline Crossref
Vaegter KK, Lakic TG, Olovsson M, Berglund L, Brodin T, Holte J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril. 2017;107:641-8.e2.
Medline Crossref
van Disseldorp J, Lambalk CB, Kwee J, Looman CWN, Eijkemans MJC, Fauser BC, Broekmans FJ. Comparison of inter- and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum Reprod. 2010;25:221-7.
Medline Crossref
van Loendersloot L, van Wely M, van der Veen F, Bossuyt P, Repping S. Selection of embryos for transfer in IVF: ranking embryos based on their implantation potential using morphological scoring. Reprod Biomed Online. 2014;29:222-30.
Medline Crossref
Van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302:1361-5.
Medline Crossref
van Swieten EC, van der Leeuw-Harmsen L, Badings EA, van der Linden PJ. Obesity and Clomiphene Challenge Test as predictors of outcome of in vitro fertilization and intracytoplasmic sperm injection. Gynecol Obstet Invest. 2005;59:220-4.
Medline Crossref
Vesco KK, Dietz PM, Rizzo J, Stevens VJ, Perrin NA, Bachman DJ, Callaghan WM, Bruce FC, Hornbrook MC. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol. 2009;114:1069-75.
Medline Crossref
Wang J, Sauer MV. In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag. 2006;2:355-64.
Medline Crossref
Wang YA, Dean J, Grayson N, Sullivan EA. Assisted reproduction technology in Australia and New Zealand 2004. Sydney: Australian Institute of Health and Welfare, National Perinatal Statistical Unit and the Fertility Society of Australia. Assisted Reproductive Technology Series; 2006. Available at: https://www.aihw.gov.au/reports/mothers-babies/assisted-reproduction-technology-australia-nz-2004/contents/table-of-contents
Wang JG, Douglas NC, Nakhuda GS, Choi JM, Park SJ, Thornton MH, Guarnaccia MM, Sauer MV. The association between anti-Müllerian hormone and IVF pregnancy outcomes is influenced by age. Reprod Biomed Online. 2010;21:757-61.
Medline Crossref
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77-83.
Medline Crossref
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, Pera RAR. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115-21.
Medline Crossref
World Health Organization (WHO). Health statistics and in- formation systems. Maternal mortality ratio (per 100 000 live births). Geneva: WHO; 2019a. Available https://www.who.int/healthinfo/statistics/indmaternalmortality/en/#
World Health Organization (WHO). Body mass index - BMI. Geneva: WHO; 2019b. Available at: http://www.euro.who.int/en/health-topics/disease-prevention/nutri- tion/a-healthy-lifestyle/body-mass-index-bmi
Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, do Carmo Borges de Souza M. Assisted reproductive technologies (ART) in Latin America: The Latin American Registry, 2012. JBRA Assist Reprod. 2014;18:127-35.
Crossref
Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, do Carmo Borges de Souza M. Assisted reproductive technologies in Latin America: the Latin American Registry, 2012. Reprod Biomed Online. 2015;30:43-51.
Medline Crossref
Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, Liu F, Li M, Sun S, Xing L, Zhu Y, Chen Y, Xu L, Zhou L, Huang H, Zhang D. Maternal and Live-birth Outcomes of Pregnancies following Assisted Reproductive Technology: A Retrospective Cohort Study. Sci Rep. 2016;6:35141.
Medline Crossref
Zhu Q, Chen Q, Wang L, Lu X, Lyu Q, Wang Y, Kuang Y. Live birth rates in the first complete IVF cycle among 20,687 women using a freeze-all strategy. Hum Reprod. 2018;33:924-9.
Medline Crossref